The stress sigma factor of RNA polymerase RpoS/σ S is a solvent-exposed open molecule in solution
Résumé
In bacteria, one primary and multiple alternative sigma (σ) factors associate with the RNA polymerase core enzyme (E) to form holoenzymes (Eσ) with different promoter recognition specificities. The alternative σ factor RpoS/σ S is produced in stationary phase and under stress conditions and reprograms global gene expression to promote bacterial survival. To date, the three-dimensional structure of a full-length free σ factor remains elusive. The current model suggests that extensive interdomain contacts in a free σ factor result in a compact conformation that masks the DNA-binding determinants of σ, explaining why a free σ factor does not bind double-stranded promoter DNA efficiently. Here, we explored the solution conformation of σ S using amide hydrogen/deuterium exchange coupled with mass spectrometry, NMR, analytical ultracentrifugation and molecular dynamics. Our data strongly argue against a compact conformation of free σ S. Instead, we show that σ S adopts an open conformation in solution in which the folded σ 2 and σ 4 domains are interspersed by domains with a high degree of disorder. These findings suggest that E binding induces major changes in both the folding and domain arrangement of σ S and provide insights into the possible mechanisms of regulation of σ S activity by its chaperone Crl.
Fichier principal
 Manuscript Cavaliere et al.1.pdf (414.15 Ko)
Télécharger le fichier
Manuscript Cavaliere et al.1.pdf (414.15 Ko)
Télécharger le fichier
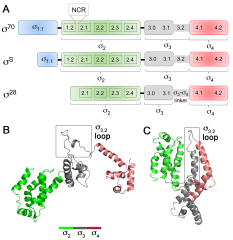 Figure 1.tif (11.49 Mo)
Télécharger le fichier
Figure 1.tif (11.49 Mo)
Télécharger le fichier
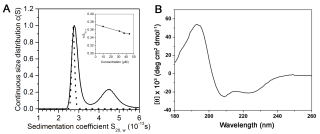 Figure 2.tif (8.87 Mo)
Télécharger le fichier
Figure 2.tif (8.87 Mo)
Télécharger le fichier
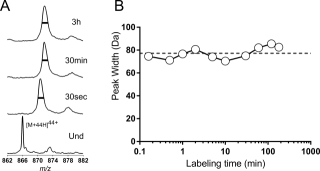 Figure 3.tif (247.59 Ko)
Télécharger le fichier
Figure 3.tif (247.59 Ko)
Télécharger le fichier
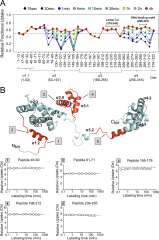 Figure 4.tif (1.09 Mo)
Télécharger le fichier
Figure 4.tif (1.09 Mo)
Télécharger le fichier
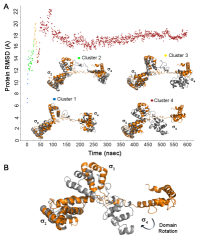 Figure 5.tif (988.37 Ko)
Télécharger le fichier
Supplementary data Cavaliere et al.pdf (3.37 Mo)
Télécharger le fichier
Figure 5.tif (988.37 Ko)
Télécharger le fichier
Supplementary data Cavaliere et al.pdf (3.37 Mo)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image



