Polo-like kinase 1 (PLK1) regulates IFN induction by MAVS.
Résumé
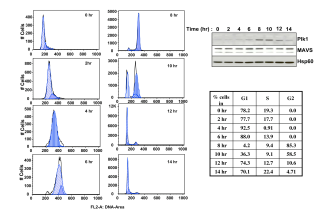
The mitochondria-bound adapter MAVS participates in IFN induction by recruitment of downstream partners such as members of the TRAF family, leading to activation of NF-kappaB, and the IRF3 pathways. A yeast two-hybrid search for MAVS interacting proteins yielded the Polo-box domain (PBD) of the mitotic Polo-like kinase PLK1. We showed that PBD associates with two different domains of MAVS in both dependent and independent phosphorylation events. The phospho-dependent association requires the phosphopeptide binding ability of PBD. It takes place downstream of the Proline-rich domain of MAVS, within an STP motif, characteristic of the binding of PLK1 to its targets, where the central T234 residue is phosphorylated. Its phospho-independent association takes place at the C-terminus of MAVS. PLK1 strongly inhibits the ability of MAVS to activate the IRF3 and NF-kappaB pathways and to induce IFN. Reciprocally, depletion of PLK1 can increase IFN induction in response to RIG-I/SeV or RIG-I /poly(I)-poly(C) treatments. This inhibition is dependent on the phospho-independent association of PBD at the C terminus of MAVS where it disrupts the association of MAVS with its downstream partner TRAF3. IFN induction was strongly inhibited in cells arrested in G2/M by nocodazole, which provokes increased expression of endogenous PLK1. Interestingly, depletion of PLK1 from these nocodazole-treated cells, could restore, at least partially, IFN induction. Altogether, these data demonstrate a new function for PLK1 as a regulator of IFN induction and provide the basis for the development of inhibitors preventing the PLK1/MAVS association in order to sustain innate immunity.
Domaines
Immunité innée
Fichier principal
 suppl.pdf (1.36 Mo)
Télécharger le fichier
vitour_et_al.pdf (4.06 Mo)
Télécharger le fichier
suppl.pdf (1.36 Mo)
Télécharger le fichier
vitour_et_al.pdf (4.06 Mo)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)